Numerous molecular cloning techniques are currently available that allow for the insertion of a specific DNA fragment, or in some cases more than one fragment, into a vector of choice. Some of these cloning methods employ a restriction enzyme, which recognizes specific base pair sequence and cuts both DNA strands on both the vector and insert to create compatible ends, allowing the two to be joined together by DNA ligase. One of these methods is Golden Gate cloning.
What is Golden Gate cloning?
Golden Gate cloning utilizes Type IIS restriction enzymes to cut the insert and vector outside of their recognition site allowing for several fragments to then be assembled together with the vector in one reaction while at the same time also avoiding the formation of a scar in the final construct [1]. The overhangs produced using a Type IIS restriction enzyme are independent of the recognition site and unique to the sequence of the DNA fragments so after ligation no residual bases of the recognition sequence will be present in the cloned construct, preventing a so-called recognition site scar [1].
Because different Type IIS restriction enzymes cut the DNA at different locations outside of their recognition site, overhang sequences of differing lengths can be produced. By carefully designing the sequences based on the IIS restriction enzymes to be used, multiple adjacent fragments can be assembled together into a vector in a single ligation reaction [2]. To accomplish this, it is important to design DNA fragments with Type IIS recognition sites at the terminal ends that overlap with the adjacent fragments.
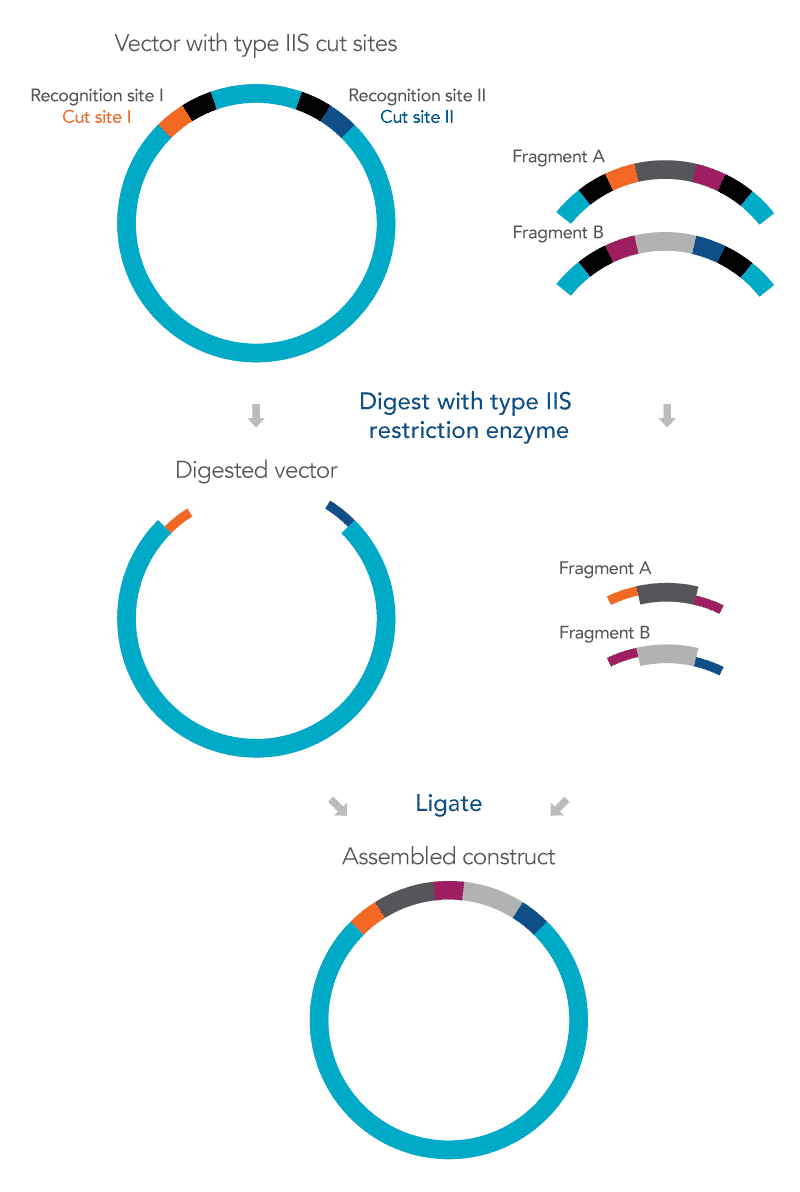
Figure 1. An overview of the Golden Gate cloning method. The Golden Gate cloning method inserts more than one target DNA sequence into a vector. The vector and the DNA fragments, designated as fragments A and B, contain recognition sites (black) and cut sites (orange, navy blue, as well as purple to join the two fragments together). Both vector and DNA fragments are digested with Type IIS restriction enzymes, which generate a short nucleotide sequence overhang. The digested vector and the DNA fragments do not include the recognition sites, as shown in the figure. The overhang regions (designated as orange, navy blue, and purple in the figure) ligate to form an assembled construct.
Key benefits of Golden Gate cloning
Golden Gate cloning offers a powerful and efficient solution for assembling multiple DNA fragments with precision and speed, making it ideal for complex and modular genetic constructs.
- Scarless assembly: Joins fragments without leaving extra bases
- Multi-fragment capability: Efficiently assembles multiple inserts in one reaction
- One-pot reaction: Combines digestion and ligation in a single step
- High precision: Type IIS enzymes allow controlled orientation and order of fragments
- Ideal for modular cloning: Great for building complex constructs like pathways or libraries
What is the Standard Golden Gate cloning protocol?
Golden Gate cloning streamlines multi-fragment assembly using Type IIS enzymes and a one-pot digestion-ligation reaction.
- Design fragments with flanking Type IIS sites (e.g., BsaI) and compatible overhangs.
- Set up reaction with vector, inserts, buffer, and Golden Gate Assembly Mix.
- Run thermocycling by alternate between digestion (37°C) and ligation (16°C), followed by a final incubation at 60°C.
Applications of Golden Gate cloning
Golden Gate cloning is scarless and can be used for cloning single inserts, assembling large constructs originating from multiple fragments, as well as assembling fragments that contain internal structural complexities [1]. It is considered an efficient method that can be done in a single tube and, as it works better than some other methods with a wide range of fragment lengths, it lends itself to different levels of assembly. For example, it can be used to assemble several short genetic elements together to create a single gene, such as promoters, open reading frames, and terminators, or can be used to rapidly construct multiple genes into a vector simultaneously. It can even be used in to generate multigene constructs from a library of assembled genes [3].
Considerations and limitations of Golden Gate cloning
Areas that will not work well for Golden Gate cloning include sequences that contain GC extremities or secondary structures in the overlap regions. Although it can work better with repeat sequences than other scarless cloning methods that rely on homology alone, for Golden Gate assembly to work, it is critical that the Type IIS enzyme recognition site is uniquely present in the cloning site and does not appear anywhere else in the DNA sequence. If the recognition site occurs anywhere else in the insert or the vector, it will need to be removed to prevent undesired cuts. If there is an overhang with similar sequences (e.g., only 1 base pair difference) there is the chance that the vector may re-ligate which will decrease the cloning efficiency.
Design tips for Golden Gate cloning
Include Type IIS enzyme cut sites
- Add the cut sequence of the Type IIS enzyme to the terminal regions of the insert.
- Use 4-base overhangs for highest accuracy.
- Ensure the recognition site is placed terminal to the cut site.
- Add the cut sequence of the Type IIS enzyme to the terminal regions of the insert.
- Use 4-base overhangs for highest accuracy.
- Ensure the recognition site is placed terminal to the cut site.
Spacer sequences may be required
Some enzymes need a spacer between the recognition and cut sites:
- If the enzyme cuts 1 bp outside the recognition site → add 1 bp spacer.
- If it cuts 2 bp outside → add 2 bp spacer, and so on.
Add flanking sequences
- Include 4–5 bp flanking sequences on both ends of the fragment.
- These help recruit enzymes and increase reaction efficiency.
Use plasmids as starting material
- Plasmids can be used as starting material for Golden Gate cloning reactions.
- IDT offers cloning vectors free of Type IIS sites outside the cut site.
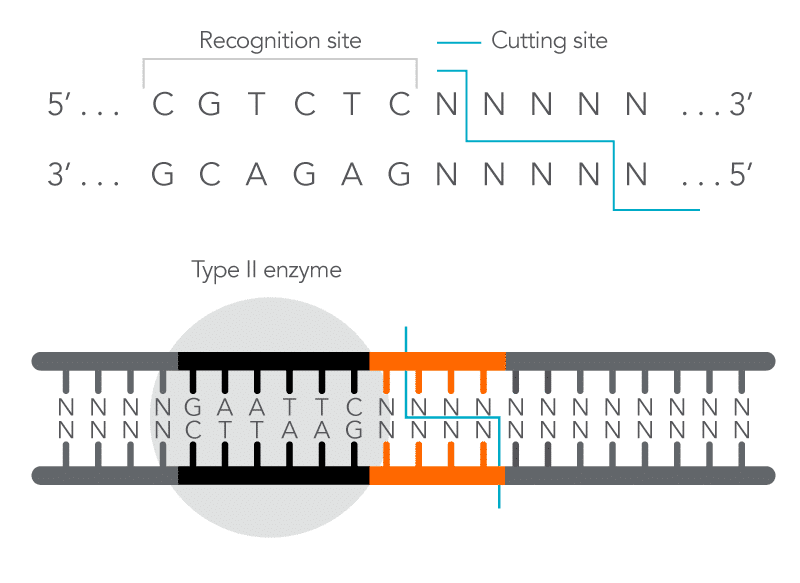
Figure 2. The restriction enzyme’s recognition site differs from its cutting site. A Type IIS restriction enzyme cuts the non-specific nucleotide sequence away from its recognition site in the DNA molecule.
References
- Bird JE, Marles-Wright J, Giachino A. A User's Guide to Golden Gate Cloning Methods and Standards. ACS Synth Biol. 2022;11(11):3551-3563.
- Engler C, Marillonnet S. Golden Gate cloning. Methods Mol Biol. 2014;1116:119-131.
- Grützner R, Marillonnet S. Generation of MoClo Standard Parts Using Golden Gate Cloning. Methods Mol Biol. 2020;2205:107-123.
RUO23-2354_001.1























